

Our mission is to exploit developmental, genetic and cellular information to better understand inherited and age related retinal disease and to work towards new treatments for patients with retinal disease.
Studying the developmental biology and disease of the eye is a core activity of the Retinal Stem Cell Research group (RSCR), led by Prof. Majlinda Lako in the Biosciences Institute at Newcastle University.
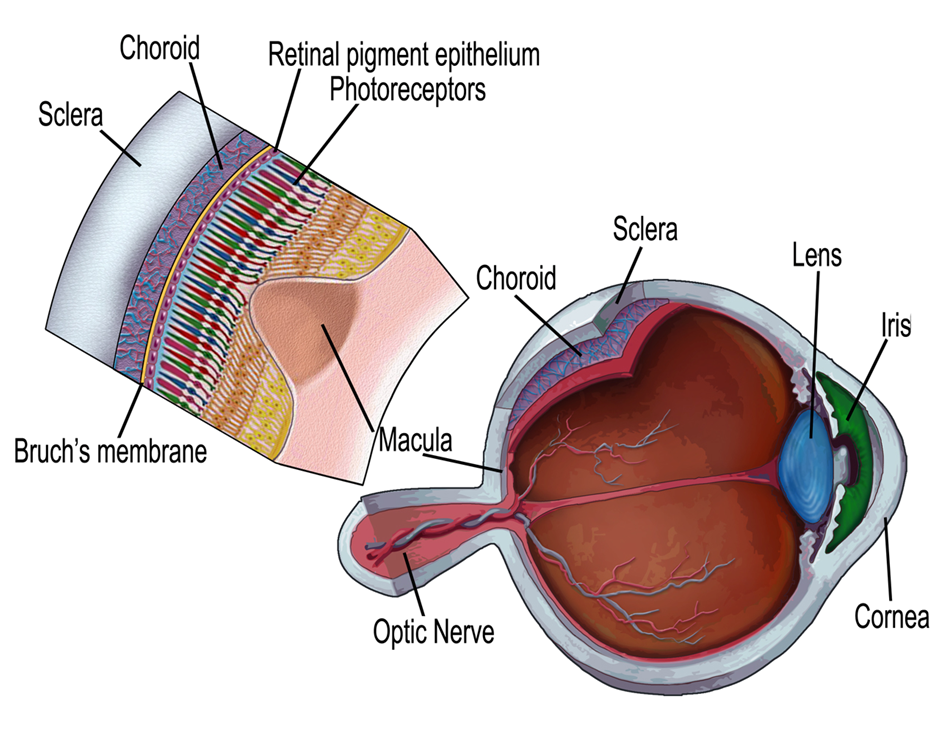
The retina is a thin sheet of central nervous tissue which covers the back of the eye and is responsible for converting light into electrochemical signals that can be transmitted to the brain via the optic nerve, whereupon they will be processed to give the sensation we understand as vision.
The light conversion process begins when light enters the eye through the pupil and passes through the lens which causes it to focus on the retina. When the light shines on specialised cells known as photoreceptors, it causes pigments called rhodopsin/opsin to trigger a cascade of chemical changes that cause the cell to lose sodium ions and become "polarised".
This is a bit like a chemical battery - the polarised cells now have enough potential energy to transmit a signal to the next group of cells that in turn will pass this signal to the cells that form the optic nerve. This is a complex process, which of course means that there are lots of opportunities for things to go wrong.
Dysfunction of any of the cell types that reside in the retina or supporting cells, the retinal pigmented epithelium (RPE), which is vital for the health of the retina, will disrupt vision - our aim is to determine the mechanisms that cause such disruption and find ways to repair the damage.
A schematic diagram of the retina (Mellough et al. Stem Cells. 2009 Nov;27(11):2833-45).
Pluripotent stem cells are able to grow indefinitely while retaining the ability to turn into any of the cell types found in the adult body.
A few years ago, the only way we could obtain cells with these characteristics was, with appropriate patient consent, to extract embryonic stem cells from spare human embryos created for the purpose of in vitro fertilisation, but that were no longer needed. These days we don't need to do this since we can obtain cells with embryonic stem cell-like properties from many adult cell types via a process called reprogramming.
We can perform this technique on adult cells which can be easily collected from patients; a few millilitres of blood or even a few hairs are all we need to make these reprogrammed cells, which are called "induced pluripotent stem cells" (iPSC for short). Just like their embryonic stem cell counterparts, these will grow indefinitely and can turn into any type of adult cell we want.
Naturally, this includes most of the cells of the retina, so iPSCs are a great resource for learning more about how the retina is assembled in the embryo - but also for understanding how retinal disease can occur. We can do this by making iPSCs from patients affected by retinal disease and examining the genetic and functional differences between retinal cells made from the patient's iPSCs and those made from iPSCs which were created from healthy donors.

Retinal Stem Cell Research
Biosciences Institute
Newcastle University, International Centre for Life, Central Parkway, Newcastle upon Tyne, NE1 3 BZ. United Kingdom
Tel: +44 (0)191 241 8688
Email: majlinda.lako@ncl.ac.uk








